The ENCePP Guide on Methodological Standards in Pharmacoepidemiology was created and is maintained by members of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP)–specifically, the ENCePP Working Group 1 on Research Methods and Guidance, chaired by RTI Health Solutions’ Alejandro Arana.
It provides methodological guidance in pharmacoepidemiology to researchers, clinicians, marketing authorization holders and applicants, and regulators worldwide. The newest version, Revision 9, has been released this month.
The Guide is dynamic in nature and updated every year. It is the most used deliverable of ENCePP with more than 10,000 views and around 2,000 downloads per month. This year’s revisions were quite extensive. The revision team selected studies to illustrate relevant methodologies and completed a structural reorganization. The most viewed topics during 2020 were on methods to address bias and confounding and study design.
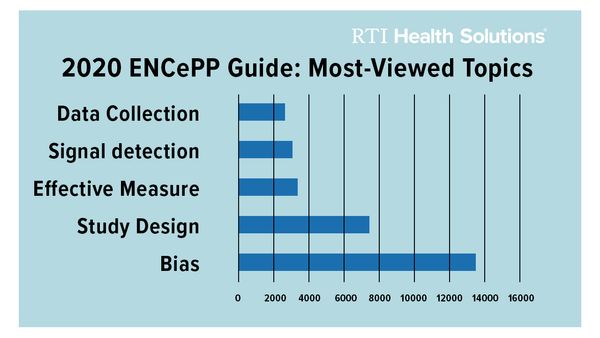
Graph Source: ENCePP Activity Report January-December 2020
The COVID-19 pandemic and ensuing vaccination campaigns have caused timelines for the completion of studies and publication of results to be compressed. Susana Perez-Gutthann of RTI Health Solutions and Gianmario Candore, Co-Chairs of the ENCePP Steering Group, shared, “ENCePP is confident that this 9th revision of the ENCePP Guide will contribute to the use of appropriate methods in COVID-19 research, to the conduct of valid and reliable studies on the effectiveness and safety of treatments and vaccines and, ultimately, to the effective management of the COVID-19 pandemic.”
RTI Health Solutions’ Susana Perez-Gutthann and Alejandro Arana have contributed to several guidance sections, including Approaches to Data Collection, Study Design, and Methods to Address Bias and Confounding.
ENCePP Guide on Methodological Standards in Pharmacoepidemiology
